1. Distinguish between Avogadro’s number and the mole.
A mole is a counting unit. Other counting units you may be familiar with are dozen and pair. We know that “dozen” means “12”. We can use dozen for anything: a dozen eggs are 12 eggs. 3 dozen eggs are 36 eggs. We know that pair means 2. We can use pair for anything: a pair of tickets means 2 tickets.
A mole simply means 6.022 x 1023. Like “dozen” and “pair”, we can use “mole” for anything. A mole of marbles would be 6.022 x 1023 marbles. That’s 6 trillion billion hundred marbles. This is enough marbles to cover the entire Earth over 1 mile deep!
Although we can use a mole to count anything we want, we usually only use it in Chemistry to count atoms or molecules.
Pair: 2
Dozen: 12
Mole: 6.022 x 1023
Avogadro’s number is 6.022 x 1023. Avogadro’s number is to the mole what “12” is to a “dozen”, or what 2 is to a “pair”.
2. Define
a) percentage composition
The percent composition of a
component in a compound is the percent of the total mass of the
compound that is due to that component.
Consider a water molecule, H2O. It has twice as many hydrogen atoms as it does oxygen atoms. So you might be tempted to think that hydrogen makes up a greater percentage of a water molecule than oxygen. But a mole of hydrogen atoms is only 1 gram. (Look at the atomic mass on the periodic table. It’s the number below each element’s symbol.) A mole of oxygen atoms is 16 grams. Each water molecule has 2 hydrogen atoms and 1 oxygen atom. So a mole of water molecules will contain 2 moles of hydrogen atoms and 1 mole of oxygen atoms. H2O will have a molar mass of 2(1 g/mol) + 1(16 g/mol) = 18 g/mol. Oxygen accounts for 16 of these 18 grams, therefore the percent composition of oxygen is 16/18 = 0.89 or 89%, while the hydrogen atoms account for only 11%.
b) empirical formula
The empirical formula of a compound is the simplest integer ratio of atoms of each element in a compound.
c) molecular formula
A molecular formula shows the atoms and
the number of atoms in a molecule. For
example, CO2 shows us that carbon dioxide has 1 carbon atom and 2
oxygen atoms.
d) average atomic mass
Average atomic mass is a weighted average of all the isotopes of an atom.
3) How many moles of magnesium oxide are there in 2.5 x 1025 molecules of MgO?
![]()
4) How many moles are equal to 3.6 x 1023 molecules of oxygen gas, O2?
![]()
5) How many grams are present in 4.336 x 1024 molecules of table salt, NaCl, whose molar mass is 58.4 g/mol?
![]()
6) Calculate the mass in grams of 2.55 mol of oxygen gas, O2.
![]()
7) How many grams are in each of the following samples? (molar mass should be to one decimal point (tenths place).
a) 1.00 mol NaCl
![]()
b) 2.00 mol H2O
![]()
c) 3.5 mol Ca(OH)2
![]()
8) How many atoms of gold are there in a pure gold ring with a mass of 10.6 g? (make sure molar mass is to the tens place).
![]()
9) Find the molar mass of the following compounds
a) KNO3
K: 1(39.1 g/mol)
N: 1(14.0 g/mol)
O: 3(16.0 g/mol)
=101.1 g/mol
b) Na2SO4
Na: 2(23.0 g/mol)
S : 1(32.1 g/mol)
O : 4(16.0 g/mol)
=142.0 g/mol
c) Al2(CrO4)3
Al: 2(27.0 g/mol)
Cr: 3(52.0 g/mol)
O : 12(16.0 g/mol)
=402 g/mol
10) Find the molar mass of the following compounds (first you must put together the compound.
a) lithium chloride
Put together the compound: Li+1Cl-1 . Criss-cross to get: LiCl
So lithium chloride has 1 lithium atom and 1 chlorine atom.
Li: 1 (6.9 g/mol)
Cl: 1 (35.5 g/mol)
=42.4 g/mol
b) magnesium nitrate
Put together the compound
Mg+2NO3-1 criss-cross: Mg(NO3)
So magnesium nitrate has 1 magnesium atom, 1 nitrogen atom and 3 oxygen atoms.
Mg: 1 (24.3 g/mol)
N: 1 (14.0 g/mol)
O: 3 (16.0 g/mol)
=86.3 g/mol
c) iron(III) hydroxide
Put together the compound
Fe+3OH-1 . Criss-cross to get the: Fe(OH)3
Fe: 1 (55.8 g/mol)
O : 3 (16.0 g/mol)
H: 3 (1.0 g/mol)
=74.8 g/mol
11) A compound of silver has the following percent composition: 63.5% Ag, 8.3% N, and 28.3% O. Calculate the empirical formula.
Pretend you have 100 grams (it makes the math much easier, because your percentages turn into grams)
Convert each element into grams and divide by its molar mass
Ag: 63.5 g / (107.0 g/mol) = 0.593 mol
N: 8.3 g / (14.0 g/mol) = 0.593 mol
O: 28.3 g / (16.0 g/mol) = 1.77 mol
So we have
Ag0.593N0.593O1.77
But we like integers, so divide each number by the lowest number. In this problem its 0.593.
Ag0.593/0.593 N0.593/0.593 O1.77/0.593
AgNO3
12) A compound of cupper is 39.8% Cu, 20.1% S, and 40.1% O. Calculate the empirical formula.
Cu: 39.8 g / (63.5 g/mol) = 0.63
S: 20.1 g / (32.1 g/mol) = 0.63
O: 40.1 g / (16.0 g/mol) = 2.5
So we have
Cu0.63N0.63O2.5
But we like integers, so divide each number by the lowest number, 0.63.
Cu0.63/0.63N0.63/0.63O2.5/0.63
CuSO4
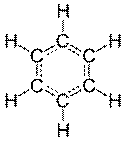
13) Benzene has the empirical formula CH and an experimental molar mass of 78 g/mol. What is its molecular formula?
We can tell by looking at a diagram of a benzene molecule that it has 6 hydrogen atoms and 6 carbon atoms. Therefore we know that its molecular formula is going to be C6H6.
But let’s work it out from the empirical formula.
The empirical formula mass of CH is
C: 1 (12.0 g/mol)
H : 1 (1.0 g/mol)
= 13.0 g/mol
But the molar mass of benzene is given in the problem to be 78 g/mol. This is 6x more than the empirical formula mass of benzene. Therefore, the molecular formula of benzene is 6 times its empirical formula:
6(CH) = C6H6
That’s the same answer we got by looking at the diagram of a benzene molecule. (You didn’t need to use diagram. I just included it to give you a sense of what we’re doing here.)
14) The empirical formula of a compound is NO2, and the molar mass is 92.0 g/mol. What is the molecular formula?
Compute empirical formula mass:
N: 1(14.0 g/mol)
O: 2(16.0 g/mol)
= 46 g/mol
Divide the molar mass by the empirical formula mass
92 / 46 = 2
So the molecular formula is 2 times the empirical formula
2(NO2) = N2O4
15) Calculate the percentage of both elements in sulfur dioxide (SO2). (% composition)
Calculate molecular mass of each element of the compound, and the molar mass of the compound itself:
S: 1(32.1) = 32.1 g/mol
O: 2(16.0) = 32 g/mol
=64.1 g/mol
So a mole of SO2 is about 64 g, and sulfur contributes 32 of these grams. So sulfur contributes half the mass, and oxygen contributes the other half.
Let’s do the math: Calculate the percentage by dividing the molecular mass of each element by the molecular mass of the compound.
32.1 / 64.1 = 0.5 = 50%
32 / 64.1 = 0.5 = 50%
Notice I multiplied by 100 to get percentage.
Sulfur: 50%
Oxygen: 50%
16) Calculate the percentage of each element in the compound of H2SO3.
H: 2 (1.0) = 2 g/mol
S: 1 (32.1) = 32.1 g/mol
O: 3 (16.0) = 48.0 g/mol
= 82 g/mol
A mole of H2SO4 has a mass of 82 grams. Hydrogen contributes 2 of these grams. Sulfur contributes 32.1 of these grams. Oxygen contributes 48 of these grams.
H: 2 / 82 = 0.024 = 2%
S: 32.1 / 82 = 0.39 = 39%
O: 48 / 82 = 0.59 = 59%
17) How many grams of Mg are in a 110 g sample of Mg(CN)2?
(Hint: Do % composition first)
Mg: 1(24.3 g/mol) = 24.3 g/mol
C : 2(12.0 g/mol) =24 g/mol
N : 2(14.0 g/mol) =28 g/mol
= 76.3 g/mol
The question only asks us about Mg. We can ignore carbon and nitrogen.
(24.3 g/mol) / (76.3 g/mol) = 0.32 = 32%
18) Isooctane has the molecular formula C8H18. What is its empirical formula?
Just reduce it like it is a fraction.
C4H9