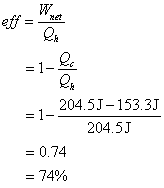1. A one-gallon bottle of hot water with a
temperature of ![]() is brought into
contact with a one-gallon bottle of cold water with a temperature of
is brought into
contact with a one-gallon bottle of cold water with a temperature of ![]() . Assuming that no
heat escapes into the environment, what will happen to the temperature of the
hot water? What will happen to the
temperature of the cold water? What will
be the final temperature of the two bottles of water.
. Assuming that no
heat escapes into the environment, what will happen to the temperature of the
hot water? What will happen to the
temperature of the cold water? What will
be the final temperature of the two bottles of water.
According to the 2nd law
of thermodynamics, heat will flow from the hot bottle to the cold bottle. The heat flow will continue until both
bottles are in equilibrium. Since both
bottles contain the same amount of water, we can simply average the temperatures
together to compute the final temperature.
Both bottles will become![]() .
.
2. A refrigerator transfers heat from the cold interior to the warmer exterior. Why doesn’t this violate the 2nd law of thermodynamics?
An external effort is involved as the refrigerator is plugged into the electrical outlet.
3. On a warm day, you decide to open the refrigerator door to cool off the kitchen. Will this work? Why or why not? Use Physics terms to explain.
No. According to the 1st Law of Thermodynamics, energy can not be created or destroyed. But it can be transferred from one form to another, and it can be transferred from one place to another. According to the 2nd law of thermodynamics, heat will only transfer from cold to hot if an external effort is applied. In other words, if work is performed on the system. The cold air in the refrigerator was created by adding energy to the system (the refrigerator) so that heat would transfer from the cold interior to the warmer kitchen air. Therefore the kitchen will warm by an amount equal to the work required to transfer heat from a cold place to a hot place.
4. A 2000 kg car is traveling at 30 m/s. What is the kinetic energy of the car? The driver steps on the brakes until the car comes to a rest. The 1st law of thermodynamics tells us that energy can not be destroyed. So where did this kinetic energy go?
![]()
It was completely converted into heat. The brake pads of the car heated up due to friction with the wheel’s rotors or drums. All the energy that was once kinetic energy is now contained in the brakes as thermal energy.
5. Find the efficiency of a gasoline engine that, during one cycle, receives 204.5 J of energy from combustion, and loses 153.3 J by heat to the exhaust.