 Physics – Exam 01 Solutions Version A
Physics – Exam 01 Solutions Version A
Name________________________________________________________________ Period________________
Circle the letter of the
answer that best completes the sentence.
(Problems 1-8 are 2 points each).
1. In an adiabatic process
A. no
heat is transferred to the environment.
B. heat
transferred to the environment is greater than heat transferred to the system.
C. internal
energy is conserved.
D. the mass of
the system increases.
E. the mass
of the system decreases.
2. In an isovolumetric process
A. the work
done to the environment is positive.
B. the volume
of the system increases.
C. the volume
of the system decreases.
D. no
work is done on the environment.
E. the system
is cooled to absolute zero.
3. In an isothermal process
A. There
is no change in temperature.
B. the volume
must remain constant.
C. the
temperature approaches absolute zero.
D. temperature
is always positive.
E. heat must
be transferred by radiation.
4. As
warm air rises, it expands and cools.
This is an example of
A. an
isothermal process
B. an
isovolumetric process
C. an
adiabatic process
D.
E. heat
transfer by conduction
 5. A rocket is an example of
5. A rocket is an example of
A. an apartment building.
B. a heat engine.
C. an
aerodynamic process.
D. an
atmosphere.
E. temperature.
6. A
barometer is designed to record changes in
A. an
adiabatic process.
B. air pressure.
C. the
1st Law of Thermodynamics.
D. the
2nd Law of Thermodynamics.
E. elevation.
7. The
temperature of a gas that is allowed to expand
A. increases.
B. decreases.
C. remains the
same.
D. is
conserved.
E. decreases
the volume of the gas.
8. All of the following are temperature scales EXCEPT
 A. Fahrenheit
A. Fahrenheit
B. Celsius.
C. Kelvin.
D. Joules.
E. All of the
above are temperature scales.
(Problems 9-14 are 4 points each)
9. Steam
moves into the cylinder of a steam engine at a constant pressure of ![]() . The diameter of the
piston is 2.0 cm. The steam does 2.5 J
of work on the piston. How far did the
piston travel?
. The diameter of the
piston is 2.0 cm. The steam does 2.5 J
of work on the piston. How far did the
piston travel?

![]() 10. A 2000 kg car accelerates from rest to10 m/s
by transferring 1 million Joules of heat to the engine from the burning of
fuel. What is the efficiency of the
engine?
10. A 2000 kg car accelerates from rest to10 m/s
by transferring 1 million Joules of heat to the engine from the burning of
fuel. What is the efficiency of the
engine?
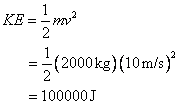
![]()
11. A gas
expands when 1000 J of energy is added to it by heat. The overall change in the internal energy of
the gas is 200 J. How many Joules of
work does the gas do on its surroundings?

 12. Briefly describe the purpose of the
refrigerant in a refrigerator. (4 sentences maximum).
12. Briefly describe the purpose of the
refrigerant in a refrigerator. (4 sentences maximum).
Read Holt, p. 313-314
13. In
what two ways can the internal energy of a system be increased according to the
1st law of thermodynamics?
Heat and work
14. An
inventor claims to have made a heat engine that is 100% efficient. Can his claim be true? Why or why not?
No. The 2nd
law of thermodynamics states that a heat engine can not transfer 100% of
thermal energy into work.